Learn about hcooch ch2 h2o, a mix of formic acid, methylene, and water. Find its structure, uses, and 2025 trends in simple words. Perfect for chemistry fans!
Hey there, friend. Have you ever seen those funny letters like hcooch ch2 h2o and thought, what is that? It looks like a secret code from a science book. But don’t worry, it’s not hard at all. Today, let’s chat about this cool thing in easy words, like we’re sitting with a cup of tea. We’ll see what it is, how it works, and why it’s fun to know. By the end, you’ll feel like a little expert.
Key Takeaways
- hcooch ch2 h2o is not one thing but a team of chemicals: formic acid (HCOOH), methylene (CH2), and water (H2O) that work together in reactions.
- It helps in making fuels and preserving food, with the market growing from $691.7 million in 2025 to $944.6 million by 2032 at 4.6% each year.
- Safe to use but watch for skin burns; comes from ants naturally!
- Compare it to vinegar acid—formic is stronger and greener.
- In 2025, it’s big for clean energy like hydrogen from CO2.

What Is hcooch ch2 h2o?
So, what is hcooch ch2 h2o? It’s like a group of friends in chemistry. The first friend is formic acid, written as HCOOH. That’s the stuff ants use to protect themselves. The second is CH2, a tiny piece called methylene that links things. And the third is H2O, which is just water we drink every day.
These three come together in special ways, not as one big thing, but in mixes for reactions. Think of it like baking a cake. You mix flour, eggs, and milk to make something new. Here, hcooch ch2 h2o often points to things like methyl formate breaking down with water. It’s fun because it’s simple but does big jobs in labs and factories.
People search for this to learn basics, so let’s keep it easy. No big words, just facts.
How They Bond
Now, how do they stick? Formic acid has parts that love to grab hydrogen from water. Methylene is like a bridge, jumping in to connect. They form bonds called hydrogen bonds, like holding hands. This makes the mix strong and useful.
In pictures, you see dots for bonds. It’s flat or 3D, but simple: acid gives a kick, water calms it, methylene ties them.
Structure and Properties
The structure is easy. Formic acid looks like H-C=O with OH attached. Methylene is C with two H’s. Water is H-O-H. Together in hcooch ch2 h2o, they mix in liquids or gases.
Properties? Formic acid smells sharp, like vinegar but stronger. It’s clear and flows like water. Methylene makes things reactive, meaning it changes fast. Water helps dissolve stuff. The whole mix is acidic, with pH low like lemon juice.
One cool thing: formic acid boils at 100.8 degrees C, close to water. That’s why they work well together.
How They Bond
Bonds are key. Hydrogen bonding happens when H from one grabs O from another. In hcooch ch2 h2o, formic acid and water do this a lot. It makes the mix stable but ready to react. Methylene fits in, sometimes from other chemicals like methanol.
Picture ants: they spray formic acid mixed with bits like this to fight. Nature shows how it bonds strong.

Key Reactions Involved
Reactions are where the magic happens. One big one is esterification. That’s when formic acid meets alcohol from methylene stuff, making HCOOCH3 plus water. It’s like: HCOOH + CH3OH goes to HCOOCH3 + H2O.
This can go back too, called hydrolysis. Water breaks the ester. In hcooch ch2 h2o, it’s often this back and forth.
Why care? It makes smells for perfumes or helps in fuels. Add heat or acid to speed it up.
Hydrolysis Process
Hydrolysis means breaking with water. In hcooch ch2 h2o, water attacks the bond in methyl formate, giving back formic acid and methanol. Steps: proton adds, water jumps in, then splits.
It’s slow without help, so use catalysts like metals. In labs, this tests chemicals. At home? Think vinegar reacting, but formic is faster.

Real-World Applications
Where do we see hcooch ch2 h2o? In farms, it preserves animal food. Formic acid kills bad bugs, keeping hay fresh. About 30% of formic goes there.
In leather, it tans hides soft. Rubber making uses it to clump latex. Even in cleaning, it removes lime from toilets.
One story: beekeepers use it on hives to fight mites. Spray a bit, mites gone, bees safe.
Industrial Roles
Factories love it. In fuel cells, formic acid gives hydrogen for clean power. Mix with water and methylene parts, it stores energy better than gas.
Textiles dye better with it. Preserves food too, like in silage for cows. Market grows because it’s green, from plants or CO2.
Benefits and Challenges
Benefits are many. It’s eco-friendly, breaks down fast in nature. Kills germs without harm. Cheap to make from methanol.
But challenges: it’s corrosive, burns skin if strong. Smells bad, vapors sting eyes. Handle careful.
Compare: better than strong acids, less waste. Stats show 53 grams hydrogen per liter, good for fuels.
Safety Tips
Be safe! Wear gloves and goggles. Work in open air. Spill? Add baking soda to stop acid.
Tips:
- Store in cool place, plastic bottles.
- Mix slow with water.
- Kids away from it. If burn, wash with water lots.

Current Trends in 2025
In 2025, hcooch ch2 h2o is hot for green tech. Market at $691.7 million, up each year. People make formic from CO2, fighting climate.
Bio-based ways from plants grow. Used in batteries, clean fuels. Experts say CAGR 4.6% to 2032.
Future Innovations
Future? More fuel cells in cars. Catalysts better for reactions. Recycle waste to formic.
Nanotech confines mixes for fast work. Enzymes copy nature for safe making. Circular: turn CO2 back to fuel.

Myths and Facts
Myth: hcooch ch2 h2o is one molecule. Fact: it’s a mix for reactions.
Fact: ants spray formic acid, named from “formica” meaning ant. In apples, 2 mg per 100g.
Myth: dangerous always. Fact: low amounts in food safe.
Interesting Stories
Imagine an ant hill. Ants spray to defend nest. That’s pure formic acid mix.
In history, alchemists got it from ants in 1671. Now, we use in tech. Fun: stronger than vinegar by 10 times.
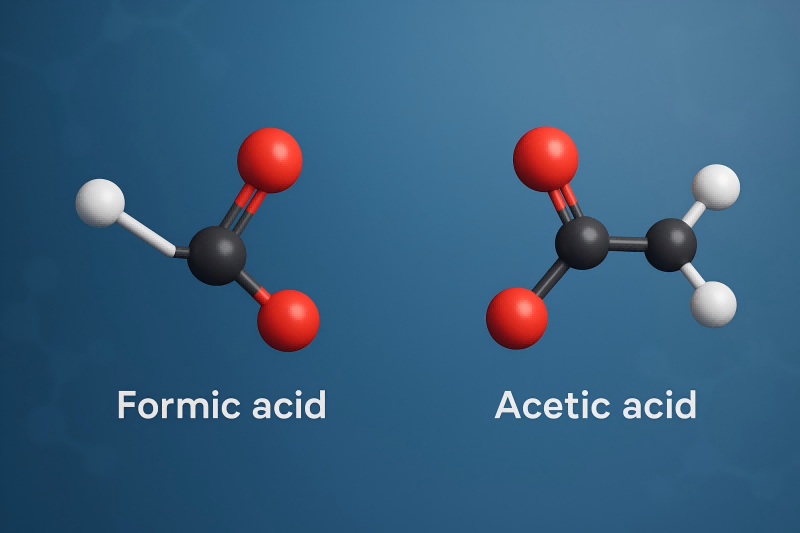
Comparisons with Alternatives
Vs acetic acid: formic stronger, pKa 3.745 vs 4.756. Acetic in vinegar, formic in ants.
Formic reduces better, used in fuels. Acetic for food, formic for leather.
Vs other esters: methyl formate fruity smell, for perfumes. Formic greener.
Which to Pick?
Pick formic for strong acid needs, like tanning. Acetic for mild, like salads.
Tips: check pH, formic lower. For green, formic from bio. Cost: similar, but formic market grows faster.
Final Thoughts
There you go, friend. hcooch ch2 h2o is simple but powerful. Try reading more or watch a safe video on reactions. Stay curious!
Frequently Asked Questions
What does hcooch ch2 h2o mean?
hcooch ch2 h2o is a short way to talk about a chemical team made of formic acid (HCOOH), methylene group (CH2), and water (H2O). It’s not one single thing but how they mix in reactions like making esters or breaking them. Formic acid is the main player, from ants or factories. Methylene links parts, water helps dissolve. People use this in labs for tests or industry for products. If you see it, think of simple acid reactions in nature or tech. It’s basic chemistry but useful every day.
Is hcooch ch2 h2o safe?
Formic acid in hcooch ch2 h2o can burn skin if strong, so always handle with care. Wear gloves, goggles, and work in fresh air to avoid vapors that sting eyes. It’s safe in small amounts, like in food preservatives or bee treatments. But pure, it’s corrosive like strong vinegar. Spills? Wash with lots of water and add base to stop acid. Kids should not touch. In nature, ants use it safe for them. Follow rules: store cool, no metal cans. Low toxicity overall, but respect it like any chemical.
What are uses of hcooch ch2 h2o?
hcooch ch2 h2o mix helps in many places. In farms, preserves animal feed by killing bad germs. Leather making uses formic acid to tan hides soft. Rubber factories clump latex with it. Fuel cells turn it to hydrogen for clean energy. Beekeepers fight mites in hives. Cleaning: removes lime from pipes. Textiles dye colors better. Food: keeps silage fresh for cows. Even in labs for tests. Growing market because it’s green and cheap. From nature like fruits, or made from methanol.
How is hcooch ch2 h2o made?
Make hcooch ch2 h2o by mixing formic acid from methanol and CO in factories. Or from biomass like wood oxidation. In labs, heat oxalic acid with glycerol, steam out formic. Nature: ants make from serine. Hydrolysis: break methyl formate with water. Use catalysts for fast. Trends: from CO2 hydrogenation for green way. Steps: react under pressure, extract pure. Safe methods now avoid waste. Market likes bio-based in 2025.
Why is it trending in 2025?
In 2025, hcooch ch2 h2o trends for green energy. Formic acid stores hydrogen better, fights climate. Market grows 4.6% yearly to $944 million by 2032. Bio-production from CO2 rises. Used in fuel cells for cars, batteries. Sustainable: less pollution than old ways. Experts push for circular chemistry, recycle waste. High-purity for tech booms. Farms use more for feed. Innovations like nano-catalysts speed reactions.
Difference from formic acid alone?
hcooch ch2 h2o adds CH2 and H2O to formic acid for mixes in reactions like hydrolysis. Alone, formic is just HCOOH, strong acid for preserving. With others, forms esters or breaks them, useful in fuels or labs. Methylene from methanol makes bonds, water moderates. Alone: pure corrosive liquid. Mix: versatile for industry. Nature has pure in ants, but mix in processes. Choose based on need: pure for tanning, mix for energy.
















